Scientists Just Discovered an “Off Switch” for Cholesterol – And It Could Save Millions of Lives

Inside every cell in your body, millions of molecular switches control life-or-death decisions. Most operate silently, maintaining the delicate balance that keeps you healthy. But scientists at the University of Texas at Arlington discovered something extraordinary: a single enzyme that, when activated, sabotages one of your body’s most essential processes.
When this molecular switch flips on, it triggers a cascade of problems that can lead to heart disease, diabetes, cancer, and dementia. Even more remarkably, researchers found they could turn it off, potentially stopping these diseases before they start.
For decades, doctors have treated cholesterol problems with medications that lower levels after damage begins. But what if they could prevent the damage entirely? What if a single treatment could address multiple diseases simultaneously by targeting their common root cause?
Body’s Hidden Switch Controls Life and Death
Your body performs countless tasks every second without conscious thought. The heart beats, lungs breathe, cells divide, and waste is cleared away. Among these essential processes, cholesterol management stands out as particularly crucial yet poorly understood by most people.
Cholesterol isn’t just about heart disease. Every cell membrane depends on proper cholesterol levels to function correctly. Your brain needs cholesterol to make hormones and neurotransmitters. Your liver uses cholesterol to produce bile acids that digest fats. When cholesterol processing breaks down, multiple organ systems suffer.
Scientists have long known that inflammation disrupts cholesterol balance, but they couldn’t explain precisely how this happens. Pieces of the puzzle existed, but the complete picture remained frustratingly unclear.
Recent research finally connected the dots. Deep inside immune cells called macrophages, a specific enzyme acts like a master switch controlling cholesterol processing. When inflammation activates this switch, it shuts down normal cholesterol uptake and triggers the disease cascade that affects millions of Americans.
When Good Cholesterol Processing Goes Wrong
Healthy cholesterol management resembles a well-orchestrated recycling system. Cells use cholesterol when needed, then send excess back to the liver for processing. Special transport proteins shuttle cholesterol between tissues, ensuring that every cell receives the appropriate amount without dangerous buildup.
Inflammation throws this system into chaos. Instead of smoothly processing cholesterol, immune cells become overwhelmed and start malfunctioning. Cholesterol begins accumulating in places it shouldn’t, particularly inside artery walls, where it forms dangerous plaques.
Understanding this process requires looking at macrophages – specialized immune cells that serve as your body’s cleanup crew. These cells typically consume cellular debris, combat infections, and preserve tissue health. They also play a crucial role in cholesterol transport, helping remove excess cholesterol from tissues.
When inflammation strikes, macrophages lose their ability to handle cholesterol effectively. Instead of clearing it away, they become foam cells filled with cholesterol deposits. These dysfunctional cells contribute to atherosclerosis, the underlying cause of heart attacks and strokes.
Meet Your Body’s Cholesterol Cleanup Crew
Macrophages earned their name from Greek words meaning “big eaters” because they consume cellular garbage. These remarkable cells patrol your bloodstream and tissues, constantly searching for threats to eliminate.
Beyond their infection-fighting duties, macrophages serve as cholesterol janitors. They express special receptors that recognize and absorb cholesterol from surrounding tissues. Under normal conditions, macrophages transport this cholesterol back to the liver for recycling or elimination.
SR-BI receptors on macrophage surfaces function like cholesterol vacuum cleaners, efficiently removing excess cholesterol from tissues. When these receptors work correctly, they prevent cholesterol accumulation and maintain healthy tissue function.
Inflammation disrupts this entire system. Macrophages become activated and start producing inflammatory signals instead of focusing on cholesterol cleanup. Their SR-BI receptors shut down, leaving cholesterol to accumulate unchecked in tissues.
Scientists Find the Molecular Troublemaker
University of Texas at Arlington researchers, led by Professor Subhrangsu S. Mandal, discovered that an enzyme called IDO1 acts as the master switch controlling macrophage cholesterol processing. When IDO1 is activated during inflammation, it produces a substance called kynurenine, which interferes with normal cholesterol uptake.
“We found that by blocking the enzyme IDO1, we are able to control the inflammation in immune cells called macrophages,” Mandal explained. “Inflammation is linked to so many conditions — everything from heart disease to cancer to diabetes to dementia. By better understanding IDO1 and how to block it, we have the potential to better control inflammation and restore proper cholesterol processing, stopping many of these diseases in their tracks.”
IDO1 breaks down tryptophan, an amino acid found in protein-rich foods. During inflammation, IDO1 production increases significantly, resulting in excessive amounts of kynurenine. High kynurenine levels signal macrophages to shut down their cholesterol-processing machinery.
Laboratory experiments confirmed this mechanism. When researchers blocked IDO1 activity, macrophages regained their ability to absorb cholesterol normally. Inflammation levels dropped, and cellular function improved across multiple measures.
How IDO1 Hijacks Your Cholesterol System

IDO1’s interference with cholesterol processing creates a destructive feedback loop. Inflammation activates IDO1, which produces kynurenine, which in turn further impairs macrophage function, thereby increasing inflammation. Breaking this cycle requires targeting IDO1 directly.
Kynurenine acts like a molecular brake pedal, slowing down cholesterol uptake when macrophages should be working harder to clear excess cholesterol. Instead of ramping up cleanup efforts during inflammation, cells reduce their cholesterol-processing capacity exactly when it’s needed most.
NF-κB, a protein complex that controls inflammatory gene expression, plays a key role in this process. When NF-κB activates, it reduces SR-BI receptor production while simultaneously increasing IDO1 activity. This coordinated response ensures that inflammation effectively shuts down cholesterol processing.
Researchers discovered they could reverse these effects by blocking IDO1. Without excessive kynurenine production, macrophages maintain their SR-BI receptors and continue absorbing cholesterol even during inflammatory episodes.
One Switch, Multiple Diseases Stopped
IDO1’s role in cholesterol processing connects it to numerous diseases beyond just heart problems. Cholesterol imbalances contribute to diabetes by affecting insulin sensitivity and pancreatic function. Cancer cells often hijack cholesterol metabolism to fuel their growth and proliferation. Dementia involves cholesterol-related changes in brain tissue.
By targeting IDO1, researchers might address multiple conditions simultaneously. Instead of treating each disease separately, doctors could prevent the underlying cholesterol disruption that contributes to all of them.
“These findings are important because we know too much cholesterol buildup in macrophages can lead to clogged arteries, heart disease and a host of other illnesses,” Mandal noted. “Understanding how to prevent the inflammation affecting cholesterol regulation could lead to new treatments for conditions like heart disease, diabetes, cancer and others.”
Cardiovascular disease alone affects over 650 million people worldwide. Diabetes impacts another 400 million. Cancer strikes millions more each year. If IDO1 inhibition could reduce the risk for even a fraction of these cases, the public health impact would be enormous.
Lab Tests Prove the Switch Really Works
Controlled laboratory experiments demonstrated IDO1’s central role in the breakdown of cholesterol processing. Researchers exposed macrophages to inflammatory triggers and observed the predicted reduction in cholesterol uptake along with increased IDO1 activity.
When they blocked IDO1 using specific inhibitors, cholesterol processing returned to normal levels despite ongoing inflammation. Macrophages regained their ability to express SR-BI receptors and absorb cholesterol from their environment.
Different inflammatory triggers produced similar results. Whether researchers used bacterial toxins or inflammatory cytokines, the pattern remained consistent: inflammation activated IDO1, IDO1 disrupted cholesterol processing, and blocking IDO1 restored normal function.
These findings suggest that IDO1 represents a common pathway through which various inflammatory stimuli disrupt cholesterol homeostasis. Targeting this single enzyme could provide broad protection against inflammation-induced cholesterol problems.
Scientists Discover a Second Troublemaker
Further investigation revealed that nitric oxide synthase (NOS) works in conjunction with IDO1 to exacerbate cholesterol processing issues. NOS produces nitric oxide, which can interfere with cellular function when generated in excessive amounts during inflammation.
Blocking both IDO1 and NOS together produced even better results than targeting either enzyme alone. This suggests that combination therapy might offer superior protection against inflammation-induced cholesterol disruption.
Having multiple therapeutic targets increases the likelihood of developing effective treatments. If IDO1 inhibition proves insufficient or causes side effects, researchers can explore NOS inhibition as an alternative or complementary approach.
What Patients Can Expect from This Discovery
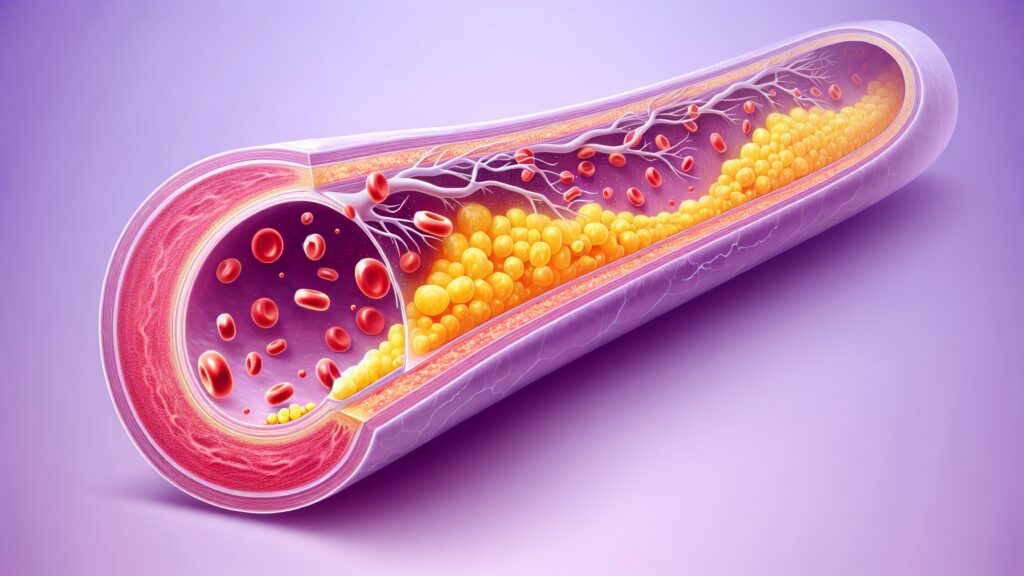
IDO1 inhibitors won’t reach patients immediately. Drug development requires extensive safety testing, dose optimization, and clinical trials to prove effectiveness in humans. Current estimates suggest that 8-12 years will pass before IDO1-targeting medications become available.
Initial clinical trials will focus on safety and dosing in healthy volunteers. If these prove successful, researchers will test IDO1 inhibitors in patients with cardiovascular disease, diabetes, or other inflammation-related conditions.
Unlike current cholesterol medications that require lifelong use, IDO1 inhibitors offer more fundamental benefits. By preventing inflammation-induced cholesterol disruption, these drugs could reduce disease risk rather than just managing symptoms.
Patients with chronic inflammatory conditions might see the most significant benefits. Rheumatoid arthritis, inflammatory bowel disease, and other autoimmune conditions involve sustained inflammation that could continuously disrupt cholesterol processing.
From Test Tube to Medicine Cabinet
Converting laboratory discoveries into approved medications involves multiple phases of testing and evaluation. Phase 1 trials establish safe dosing ranges and identify side effects. Phase 2 studies test effectiveness in specific patient populations. Phase 3 trials compare new treatments against existing standard care.
IDO1 inhibitors face unique challenges because IDO1 serves essential functions beyond cholesterol regulation. Complete IDO1 blockade may cause unintended consequences, necessitating careful dose optimization to balance the benefits and risks.
Researchers must also identify the best patient populations for IDO1 inhibition. People with active inflammation might benefit most, but determining optimal timing and duration of treatment requires extensive clinical investigation.
Revolutionary Medicine Starts with Basic Science
IDO1 research exemplifies how fundamental scientific discoveries lead to practical medical applications. By studying basic cellular mechanisms, researchers identified new therapeutic targets that could transform treatment approaches for multiple diseases.
“These findings are important because we know too much cholesterol buildup in macrophages can lead to clogged arteries, heart disease and a host of other illnesses,” Mandal emphasized. “Understanding how to prevent the inflammation affecting cholesterol regulation could lead to new treatments for conditions like heart disease, diabetes, cancer and others.”
Current cholesterol medications work downstream from the problem, lowering levels after damage has already begun. IDO1 inhibitors could work upstream, preventing the inflammatory disruption that causes cholesterol problems in the first place.
Success with IDO1 inhibition might open doors to targeting other inflammatory pathways involved in disease development. As scientists gain a deeper understanding of how inflammation is linked to various health issues, new therapeutic opportunities continue to emerge.
Future treatments might combine IDO1 inhibitors with other anti-inflammatory approaches, creating comprehensive strategies for preventing inflammation-related diseases. By targeting multiple points in the inflammatory cascade, doctors could offer more effective protection against the chronic diseases that affect millions of people worldwide.
Loading...

