A New Cancer Treatment Caused a Woman’s Tumor to Virtually Disappear in Just 5 Days
Something extraordinary happened in a hospital room at Massachusetts General that defied every medical expectation. A 57-year-old woman facing her darkest hour received an experimental treatment that would rewrite what doctors thought possible. Within 120 hours, scan results would leave medical professionals speechless.
Brain cancer had returned with a vengeance. Standard treatments had failed. Hope seemed like a luxury she could no longer afford. Yet five days after receiving a single injection of specially engineered cells, her tumor had virtually vanished from existence.
What made this possible? How did scientists achieve what seemed impossible just years ago? And why does this moment represent a turning point that could change cancer treatment forever?
Glioblastoma: Cancer That Doctors Fear Most
Glioblastoma strikes terror into even the most experienced oncologists. Among all brain cancers, this aggressive killer stands as the most deadly opponent medicine faces. Patients diagnosed with recurrent glioblastoma typically survive mere months, not years.
Standard treatments – surgery, radiation, chemotherapy – buy time but rarely deliver miracles. Tumors grow back stronger, more resistant, more determined to spread throughout the brain. Doctors watch helplessly as cancer cells hide, adapt, and multiply despite their best efforts.
Brain tumors present challenges unlike any other cancer. Protected by the blood-brain barrier, they resist many drugs that work elsewhere in the body. Surgery can remove visible masses, but microscopic cells remain scattered throughout healthy tissue. Radiation damages cancer and normal brain cells alike.
For decades, researchers have searched for better weapons against this relentless enemy. Traditional approaches target single proteins or pathways, but cancer cells evolve and find new ways to survive. Scientists needed something smarter, more adaptable, more powerful than conventional treatments.
When Traditional Medicine Meets Cutting-Edge Science
Enter CAR-T therapy – a revolutionary approach that transforms patients’ own immune cells into precision cancer hunters. Unlike chemotherapy drugs that attack broadly, CAR-T creates living medicines tailored specifically for each person’s disease.
Scientists extract T cells from patients’ blood, then engineer them in laboratories to recognize and destroy cancer. Special proteins called chimeric antigen receptors get inserted into these cells, giving them new powers to identify tumors that previously escaped immune detection.
CAR-T has already transformed blood cancer treatment, offering cures where none existed before. But solid tumors like glioblastoma presented different challenges. Cancer cells within these masses vary dramatically, displaying different proteins and characteristics. Targeting one surface marker leaves others untouched, allowing resistant cells to rebuild the tumor.
Researchers at Massachusetts General developed an innovative solution. Instead of targeting just one cancer protein, their CARv3-TEAM-E cells attack multiple targets simultaneously. These engineered T cells seek out EGFRvIII mutations while also secreting antibodies that recruit additional immune cells against wild-type EGFR proteins.
“This is a story of bench-to-bedside therapy, with a novel cell therapy designed in the laboratories of Massachusetts General Hospital and translated for patient use within five years, to meet an urgent need,” explained Dr. Bryan Choi, the neurosurgeon who helped lead this groundbreaking work.
Three Patients, Three Stories of Hope
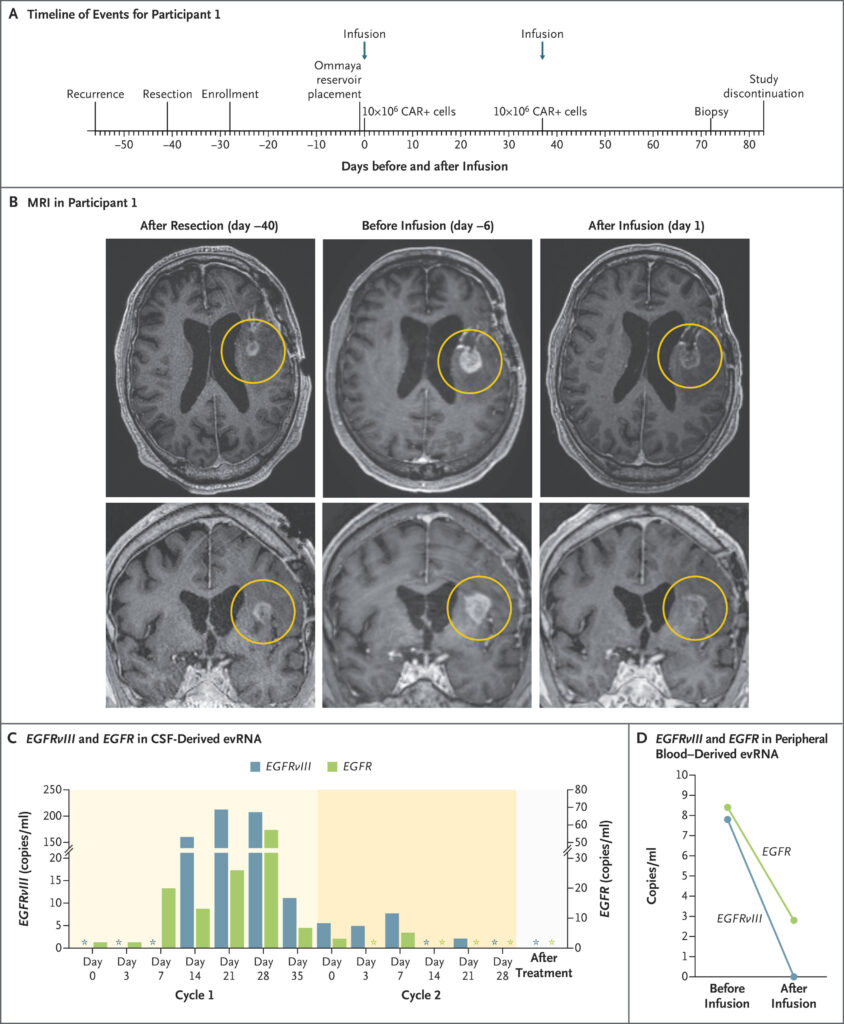
Between March and July 2023, three patients enrolled in the INCIPIENT clinical trial. Each carried the burden of recurrent glioblastoma. Each had exhausted standard treatment options. Each agreed to test this experimental approach despite unknown risks.
Patient number one was a 74-year-old man whose tumor had returned after initial treatment. Doctors removed his recurrent cancer surgically, confirming it still expressed the EGFRvIII protein their engineered cells could target. Six days before treatment, MRI scans showed active progression. But within 24 hours of receiving CARv3-TEAM-E cells, his tumor began shrinking dramatically.
Patient number two, a 72-year-old man, received his treatment after 20 months of fighting his disease. Two days post-infusion, his tumor had decreased by 18.5 percent. By day 69, it had shrunk by more than 60 percent. Most remarkably, his response continued for over six months without additional treatments.
Patient number three became the woman whose story captivated medical observers worldwide. At 57 years old, she had already battled her cancer for six months when recurrence appeared. Standard treatments had failed to control her disease. Doctors prepared her for the experimental infusion, hoping for any sign of improvement.
Engineering Immune Cells to Become Cancer Hunters
Creating these cellular weapons requires scientific precision that borders on artistry. Researchers collected each patient’s T cells through a process called leukapheresis, similar to donating blood but more selective. These cells then traveled to specialized laboratories where genetic engineering would transform them completely.
Scientists inserted new DNA sequences using lentiviral vectors, essentially reprogramming the T cells’ instructions. Multiple genetic modifications occurred simultaneously: CAR proteins to recognize EGFRvIII, antibody-producing machinery to target wild-type EGFR, and tracking markers to monitor the cells’ behavior.
Manufacturing takes weeks as modified cells multiply in carefully controlled conditions. Quality testing ensures safety and potency before cells return to patients. Each batch contains precisely 10 million CAR-positive T cells, formulated for direct injection into brain ventricles through surgically implanted Ommaya reservoirs.
Unlike systemic chemotherapy that travels throughout the body, this approach delivers treatment directly to cancer’s location. Cells enter cerebrospinal fluid surrounding the brain, accessing tumor sites while minimizing exposure to healthy organs elsewhere.
Day Zero to Day Five: A Medical Timeline
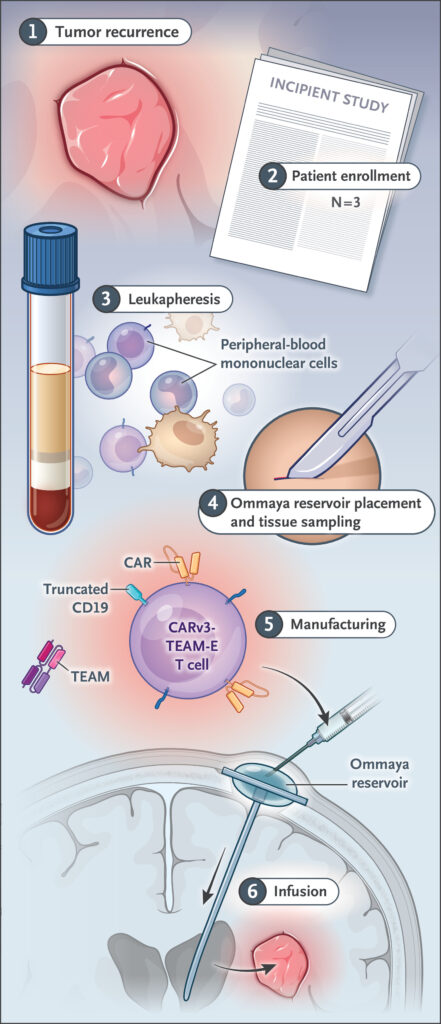
Treatment day arrived for the 57-year-old woman after careful preparation. Surgeons had previously installed an Ommaya reservoir, a small device allowing direct access to brain ventricles. Through this port, doctors would deliver millions of engineered immune cells directly to cancer’s stronghold.
Infusion itself took minutes, but anticipation filled hours. Patients receive intensive monitoring as their modified T cells begin working. Fevers develop predictably as immune systems activate. Fatigue and confusion often follow as inflammation peaks then subsides.
Day two brought encouraging signs as inflammatory markers shifted in expected patterns. Day three and four showed continued immune activation without dangerous side effects. But day five delivered results that stunned everyone involved.
“An MRI scan obtained on day 5 after this single infusion showed near-complete tumor regression,” researchers reported in their published findings. Images that had shown extensive cancer just days earlier now revealed minimal remaining disease. Her brain appeared transformed, as if the tumor had simply melted away.
When Science Meets Reality: Full Picture
Medical breakthroughs rarely arrive without complications, and this story includes sobering realities alongside its triumphs. While the woman’s five-day response amazed observers, her cancer eventually returned within one month of treatment.
Similar patterns emerged across all three patients. Initial responses proved dramatic but temporary in two cases. Only the 72-year-old man maintained his improvement beyond six months. Tumor recurrence corresponded with declining numbers of engineered T cells in cerebrospinal fluid samples.
Blood tests revealed why responses faded over time. CAR-T cells peaked around day 21 but declined rapidly afterward. Without sustained immune pressure, remaining cancer cells regrouped and rebuilt tumors. Success required not just initial tumor destruction but lasting immune surveillance.
Liquid biopsy techniques allowed researchers to track cancer DNA in cerebrospinal fluid and blood samples. Initially elevated levels of EGFRvIII and EGFR genetic material dropped to undetectable ranges after treatment, confirming that engineered cells had eliminated their targets effectively.
Massachusetts General’s Bench-to-Bedside Achievement

Behind these remarkable results lies years of dedicated research and unprecedented collaboration. Dr. Marcela Maus built a team capable of translating laboratory discoveries into human treatments within just five years – lightning speed for medical research.
“We haven’t cured patients yet, but that is our audacious goal,” Maus acknowledged while celebrating early successes. Her laboratory invested heavily in developing infrastructure needed to advance experimental therapies rapidly from concept to clinical testing.
Mass General’s Gene and Cell Therapy Institute provided essential support, helping researchers navigate complex regulatory requirements and manufacturing challenges. Multidisciplinary teams included neurosurgeons, oncologists, immunologists, and cell therapy specialists working together seamlessly.
Manufacturing occurred through the Connell and O’Reilly Families Cell Manipulation Core Facility, demonstrating how institutional investment in cutting-edge infrastructure enables breakthrough treatments. Without these resources, laboratory discoveries might have remained trapped in research journals rather than reaching desperate patients.
Beyond Individual Success: What This Means for Cancer Treatment
While three patients cannot prove effectiveness conclusively, their responses suggest transformative potential for cancer medicine. CAR-T therapy has already revolutionized blood cancer treatment, offering cures where none existed previously. Solid tumors represent the next frontier.
Glioblastoma’s resistance to conventional treatments makes it an ideal testing ground for innovative approaches. Success here could translate to other solid cancers that similarly evade current therapies. Breast, lung, pancreatic, and ovarian cancers might all benefit from dual-targeting strategies.
Personalized cell therapy represents medicine’s most individualized approach – each treatment uses patients’ own cells engineered specifically for their disease. No two cancer cases are identical, so treatments must adapt accordingly. CAR-T offers that flexibility.
Direct injection methods could apply beyond brain cancers. Tumors in lungs, liver, or other organs might respond to locally delivered engineered immune cells. Systemic toxicity decreases when treatments stay concentrated where cancer cells lurk.
Making Miracles Sustainable
Current results prove that dramatic responses are possible, but durability remains the ultimate challenge. Researchers are already planning modifications to extend treatment benefits beyond initial tumor destruction.
Serial infusions might maintain immune pressure longer than single treatments. Like booster shots for vaccines, repeated CAR-T doses could prevent cancer recurrence. Timing and frequency require careful optimization to balance effectiveness against toxicity.
Chemotherapy preconditioning might help engineered cells persist longer in patients’ bodies. By temporarily suppressing normal immune function, doctors could give CAR-T cells more time to establish themselves and maintain anti-cancer surveillance.
Combination approaches pairing CAR-T with checkpoint inhibitors, vaccines, or other immunotherapies might create synergistic effects. Cancer requires multiple simultaneous attacks to prevent escape and resistance.
Medicine stands at a crossroads where individual patient stories like the 57-year-old woman’s five-day transformation could reshape how we approach humanity’s oldest enemy. Her tumor may have returned, but her brief victory proved that even the most aggressive cancers can be defeated. Now scientists must learn to make those victories permanent.
Loading...

