Chinese Scientists Say They Created a Cure for Type 1 Diabetes
What if the cure for one of the world’s most relentless chronic diseases wasn’t in a pill or a pump — but inside your own body, waiting to be reawakened?
For over a century, Type 1 diabetes has meant a lifetime tethered to insulin injections, glucose monitors, and the quiet fear of what might happen if either slips out of balance. Every bite, every workout, every night’s sleep is shadowed by the question: Will my blood sugar hold steady? Yet despite decades of research, a true cure remained out of reach until now.
In a medical first that’s catching the attention of scientists around the globe, a team of Chinese researchers may have done the unthinkable: they’ve reversed Type 1 diabetes in a human being. A 25-year-old woman, once dependent on daily insulin, now produces it on her own thanks to a revolutionary stem cell therapy made from her own cells. No donor, no match, no miracle drug. Just her biology, reprogrammed to remember what it once knew.
It sounds like science fiction. But it’s real. And it could reshape how we think about chronic illness, the future of regenerative medicine, and what the word incurable truly means.
The First Reversal of Type 1 Diabetes
In 2023, something extraordinary happened in Tianjin, China something that might one day be seen as a turning point in medical history.
A 25-year-old woman, living with Type 1 diabetes, stopped needing insulin. Not because of a new medication or a high-tech device, but because her own body started producing insulin again. After receiving a groundbreaking stem cell transplant, she became the first person in the world whose Type 1 diabetes was reversed using cells derived from her own body.
The procedure was the result of years of research led by Dr. Deng Hongkui and his team at Peking University. Using a technique involving chemically induced pluripotent stem cells (CiPSCs), the scientists reprogrammed the woman’s adult cells into stem cells capable of becoming insulin-producing beta cells the very type that her immune system had destroyed. These reprogrammed cells were then formed into three-dimensional clusters known as islets and transplanted into her abdominal muscles, a novel approach that allowed doctors to monitor them using imaging technology.
The entire transplant took less than half an hour. Within eleven weeks, her body began producing its own insulin. Blood sugar levels stabilized. Dangerous highs and lows disappeared. And the need for insulin injections a daily ritual for millions of people with Type 1 diabetes was gone.
“She remained insulin-free for over a year,” the researchers confirmed. In a conversation with Nature, the woman shared what the transformation meant in simple, joyful terms: “I can eat sugar now.”
This isn’t an isolated miracle. Early results from two other patients in the same trial also show promise, with further clinical studies planned. In Shanghai, another team used a similar technique to help a man with Type 2 diabetes stop insulin therapy further proof that stem cell-derived islet transplants could have broad-reaching implications.
Experts around the world are cautiously optimistic. Dr. James Shapiro, a leading transplant surgeon in Canada, described the results as “stunning.” Dr. Daisuke Yabe of Kyoto University called the possibility of scaling this to more patients “wonderful.”
For now, this remains early-stage research. But it has opened a door that many believed might never open. And behind it lies the possibility that diabetes, once thought incurable, may not have the last word after all.
What Makes Type 1 Diabetes So Complex
Type 1 diabetes doesn’t arrive gently. It often strikes early, in childhood or adolescence, without warning. And once it takes hold, it becomes a lifelong companion one that demands constant attention, precision, and discipline. Behind the syringes, glucose monitors, and carb counting is a simple but devastating truth: the body has turned against itself.
Unlike Type 2 diabetes, which typically develops later in life due to insulin resistance or lifestyle factors, Type 1 diabetes is an autoimmune disease. The body’s immune system designed to protect mistakenly attacks and destroys the beta cells in the pancreas that produce insulin. Without insulin, glucose cannot enter the cells to be used as energy. Instead, it builds up in the bloodstream, leading to dangerous spikes in blood sugar.
For those diagnosed, life becomes a relentless balancing act. Insulin therapy becomes non-negotiable. Every meal must be calculated. Every activity adjusted. The risk of complications from nerve damage and kidney failure to blindness and heart disease is never far behind. And it doesn’t matter how careful you are; even with the best care, the unpredictability of blood sugar levels means there’s no perfect control.
Statistics paint a sobering picture: over 1.5 million Americans live with Type 1 diabetes. Globally, it affects millions more and growing. While new technologies like insulin pumps and continuous glucose monitors have improved quality of life, they haven’t changed the underlying reality: these are management tools, not a cure.
What makes Type 1 especially difficult to treat let alone reverse is that the disease doesn’t just remove the beta cells; it programs the immune system to keep destroying them. Any attempt to replace the cells must also contend with this autoimmune hostility. That’s why even promising treatments often fall short either the new cells are attacked again, or the patient must take immunosuppressants that come with their own risks.
How the Stem Cell Therapy Works
That’s the vision behind the stem cell therapy pioneered by Chinese scientists at Peking University. Rather than relying on donor organs or synthetic drugs, researchers turned to the patient’s own biology reprogramming her cells to become exactly what her body had lost: insulin-producing beta cells. Here’s how they did it.
The process began with a technique called chemical reprogramming. Instead of using genetic manipulation which can introduce unpredictable risks the team used small molecules to convert adult cells taken from the patient into chemically induced pluripotent stem cells (CiPSCs). These stem cells have the unique ability to become almost any type of cell in the human body, including the critical islet beta cells destroyed in Type 1 diabetes.
Once the CiPSCs were created, the scientists carefully guided them to form 3D clusters of insulin-producing islet cells functionally similar to those found in a healthy pancreas. These islet clusters were then transplanted into the woman’s abdominal muscles, an unconventional but strategically chosen site. Unlike traditional islet transplants, which are often placed in the liver (where monitoring is difficult), this abdominal location allowed doctors to use MRI imaging to track the cells and, if needed, safely remove them.
The operation took less than 30 minutes.
Within just eleven weeks, the transplanted cells began functioning as intended. The woman’s blood sugar levels normalized, and she stopped needing insulin altogether. Even more impressively, she maintained over 98% of the day within target glucose range a level of control that is extraordinarily difficult to achieve through external insulin therapy.
This breakthrough builds upon foundational work by Japanese researcher Shinya Yamanaka, who first discovered how to reprogram adult cells into pluripotent stem cells in 2006, a discovery that won him the Nobel Prize. But the Chinese team advanced this science by refining the method: their chemical reprogramming technique gave them more control, fewer safety concerns, and the ability to avoid genetic modifications altogether.
By using the patient’s own cells, the therapy also reduced the risk of immune rejection, which has long plagued transplant medicine. However, because the woman had already been on immunosuppressants for a prior liver transplant, researchers could not fully assess whether the new cells would have been rejected in a typical patient. Future trials will address this by developing modified cells that can evade autoimmune attacks, even in people who are not taking immunosuppressants.
What We Know, What We Don’t Yet Know
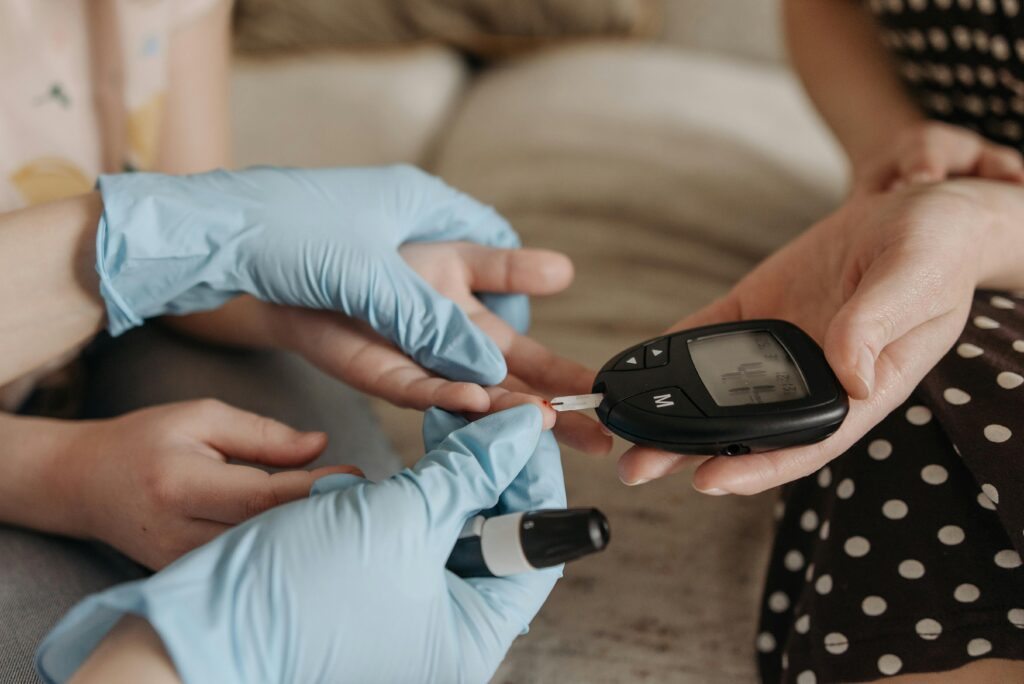
We know the treatment worked at least for one woman, and possibly others in early stages of the same clinical trial. The insulin-producing cells derived from her own body remained active for over a year. Her blood sugar control improved dramatically. And most importantly, she stopped needing external insulin something previously unimaginable for someone with Type 1 diabetes.
The science is solid. The researchers used a chemical method to induce pluripotent stem cells, avoiding the genetic risks associated with earlier techniques. The islets were carefully tested in animal models before being transplanted into the human body. And the novel implantation site in the abdomen allowed for direct monitoring of the cells a crucial safety measure rarely available in traditional liver-based transplants.
But this success, as promising as it is, opens a new set of questions that still need answers.
1. Immune Response Uncertainty
Although the cells were derived from the patient’s own tissue, the woman was already taking immunosuppressants for a previous liver transplant. This means researchers couldn’t confirm whether the reprogrammed islet cells would have been attacked by her immune system without that protection. Type 1 diabetes is, at its core, an autoimmune disease and even patient-specific cells may not be safe from an immune system primed to destroy beta cells. Future trials aim to test this in patients without prior immunosuppression.
2. Long-Term Durability
Will these new cells continue producing insulin for five years? Ten? Will they age or become dysfunctional over time? That remains unknown. As Dr. Jay Skyler from the University of Miami cautioned, one year of success is meaningful, but long-term follow-up is essential to determine whether this is a true cure or just a pause in the disease’s progression.

3. Risk of Tumor Formation
Any pluripotent stem cell therapy carries a theoretical risk of tumorigenesis. If any undifferentiated stem cells remain after the reprogramming process, they could divide uncontrollably, leading to tumor formation. So far, no abnormalities have been detected in the trial participants, but this risk underscores the need for rigorous safety protocols and continuous monitoring.
4. Variability in Patient Response
Even if future trials prove the therapy safe and effective, not every patient may respond the same way. Differences in immune profiles, age, disease severity, and genetic background could all influence outcomes. The same therapy that works for one individual might fall short for another a common challenge in personalized medicine.
5. Scale and Accessibility
The current procedure is highly personalized, time-intensive, and technically complex. It may be feasible for small groups in a research setting, but scaling this up to treat millions of patients globally is a massive undertaking. Moreover, the cost of such therapies remains prohibitively high something that could limit access, especially in lower-income regions where diabetes is rapidly rising.
Despite these challenges, experts remain optimistic. Scientists like Dr. Jagadish Hiremath acknowledge the hurdles but emphasize the potential: “Stem cell therapy is showing us that it might be possible to truly cure diseases that have long been considered only manageable and incurable.”
Can This Help Millions or Just a Few?

The therapy developed in China is highly personalized. Each treatment begins with extracting the patient’s own cells, chemically reprogramming them into pluripotent stem cells, guiding them to become insulin-producing beta cells, and then transplanting them into the body. This process requires advanced lab facilities, specialized teams, and weeks of individualized work making it both time-consuming and expensive.
Researchers and biotech companies are already exploring alternative strategies to bridge this gap. One promising approach is the use of donor-derived stem cells. In trials led by U.S.-based Vertex Pharmaceuticals, scientists have transplanted islet cells made from donated embryonic stem cells into patients with Type 1 diabetes. These early-stage patients began producing insulin again and some even became insulin-independent.
However, using donor cells reintroduces the issue of immune rejection, which means patients must take immunosuppressants drugs that suppress the body’s immune system, increasing the risk of infections and other complications. To counter this, new techniques are being tested, such as encapsulation devices protective barriers that allow insulin out but keep immune cells from destroying the transplanted islets.
Meanwhile, researchers in Japan are exploring islet sheets cell layers implanted into the abdomen, offering a surgical approach to immune protection. And in Shanghai, another team recently helped a Type 2 diabetes patient become insulin-free using similar regenerative strategies. It’s clear the momentum is global and growing.
Yet beyond science, there’s a harder question: Who will get access first?
In countries like China, where over 140 million people live with diabetes, the potential to ease the healthcare burden is enormous. If this therapy proves effective on a large scale, it could radically reduce dependence on chronic medication and reshape national healthcare strategies.
But without massive investment in infrastructure, affordability, and medical training, this cure risks becoming another innovation limited to the privileged few. The challenge now is not just perfecting the science, but ensuring it can reach those who need it most from busy city hospitals to rural clinics around the world.
A New Hope for an Old Disease
For over a century, Type 1 diabetes has meant a lifetime of vigilance needles, numbers, and the constant weight of managing what the body no longer could. It’s a disease that demands attention every waking hour, and even in sleep. For millions, the idea of a true cure has felt like wishful thinking a distant dream, not a scientific reality.
But what’s happened in China has changed the conversation.
This isn’t a vague promise or speculative theory it’s a documented case of insulin independence. A young woman, once tethered to insulin for survival, is now free. And her freedom came not from some miracle drug, but from the untapped intelligence of her own cells reprogrammed, redirected, and restored.
Yes, the work is far from over. The questions are real: long-term safety, accessibility, immune challenges, and cost. But so is the progress. What used to be considered impossible regenerating the pancreas’ lost function has now been done. Not in theory, not in animals, but in a living human being.
It’s more than just a medical achievement. It’s a signpost that says the future is not fixed. That chronic conditions, even those passed down or developed early in life, might not have to be life sentences. That science, when driven by purpose and guided by care, can do more than manage disease it can rewrite what’s possible.
So while headlines fade and research continues, one truth remains: we’ve crossed a threshold. Not just in diabetes care, but in the way we imagine healing itself.
And for the millions still waiting, still injecting, still hoping maybe, just maybe, the cure is no longer a question of “if,” but “when.”
Loading...

