Scientists just deleted the extra chromosome that causes Down Syndrome using CRISPR

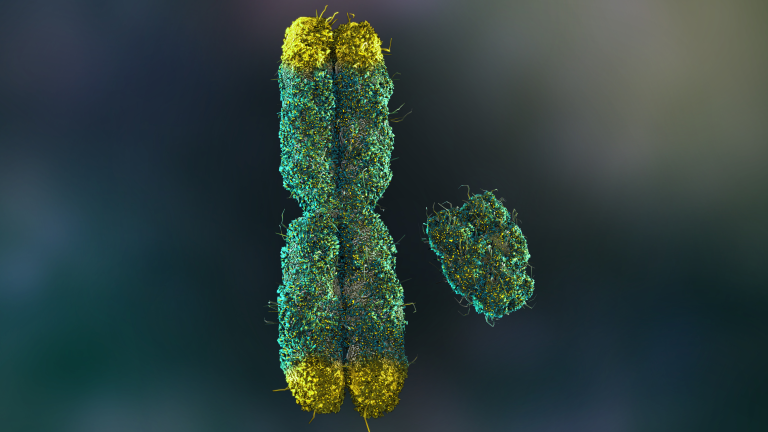
Every so often, science does something that makes us stop, look up from our phones, and wonder if the future just knocked on our door. Not with a polite tap, but with a knock so loud it shakes the frame. A team of researchers in Japan has just done something that — even a decade ago — would have sounded impossible: they used CRISPR, the gene-editing tool, to delete an entire chromosome from human cells. Not just any chromosome, but the extra copy of chromosome 21 that causes Down syndrome.
Down syndrome, or trisomy 21, is part of the human story — touching about 1 in every 700 births in the United States alone. For generations, treatments have focused on helping people live their fullest lives while managing the condition’s challenges: heart defects, learning differences, metabolic issues, and higher risks for diseases like Alzheimer’s. But the genetic root itself? That’s been untouchable. Until now.

A Leap Beyond Symptom Management
For decades, the story of Down syndrome has been told in the language of management, not reversal. Medicine could address its symptoms — offer speech therapy, perform heart surgeries, support learning — but the genetic signature itself, an extra copy of chromosome 21, remained untouchable. That extra chromosome is more than a microscopic anomaly; it alters how the body’s cells work from the inside out, influencing brain development, heart function, metabolism, and even the risk for Alzheimer’s disease.
Now, a research team in Japan has done something once thought impossible. Led by Ryotaro Hashizume at Mie University, scientists used the CRISPR-Cas9 gene-editing tool to remove the surplus chromosome in human cells grown in the lab. Unlike traditional treatments, which work around the genetic overload, this approach strikes at its source — a process known as “trisomic rescue.”
CRISPR-Cas9 works like a molecular scalpel, guided by a custom-built “map” to the exact DNA sequence it needs to target. In this case, researchers designed their system to home in only on the extra chromosome, leaving the healthy pair untouched. The results were striking: once freed from the genetic excess, the edited cells returned to more typical patterns of gene activity and protein production. Genes linked to brain development became more active, while overactive metabolic genes calmed down.
For the first time, scientists weren’t just altering a single faulty gene — they had deleted an entire chromosome. It’s a proof-of-concept that expands what gene editing can even mean. Still, this is a laboratory milestone, not a clinical cure. The breakthrough lies not in an immediate therapy, but in the widening of what’s possible.
How Editing Changed the Cells
After the extra chromosome was removed, the altered cells began behaving differently — and in ways that suggested they were healthier. The corrected cells grew faster and divided more efficiently than their trisomy counterparts, a sign that the biological strain of carrying excess genetic material had eased. This was not just a cosmetic improvement; at the molecular level, the cells were making fewer reactive oxygen species, harmful byproducts linked to cellular stress, inflammation, and aging. Lowering this oxidative burden hinted at improved mitochondrial function, the powerhouse system that fuels cellular energy. In both stem cells and skin fibroblasts — mature, non-stem cells taken directly from individuals with Down syndrome — the process worked, showing that the approach could potentially benefit a wide range of cell types, not just those that are still dividing rapidly.
Just as remarkable was how the genetic “tone” of the cells shifted. Genes related to nervous system development, which tend to be underactive in Down syndrome, began to express more normally, while those tied to overactive metabolic processes slowed down. These changes line up with decades of research showing how trisomy 21 disrupts brain formation early in fetal growth and places an unusual load on other body systems. Restoring a more balanced genetic profile suggests that chromosome-level editing could influence multiple health domains at once, rather than targeting issues piecemeal. This level of correction — a wholesale return to typical gene activity — offers a glimpse into how deep the benefits of such editing might reach.
The fact that these shifts occurred even in non-dividing, mature cells is especially promising. Many of the body’s most important cells, like neurons and muscle fibers, rarely divide once they’re formed. If scientists can adapt this chromosome removal to such cells, it could open the door to interventions in tissues once thought permanently beyond reach. It moves the idea of genetic correction from something done only before birth or in rapidly growing cells to something that might one day support tissue repair and regeneration in people already living with the condition.
The Promise and the Limits of the Breakthrough
This achievement stands as a testament to how far genetic science has come. A decade ago, deleting an entire chromosome from human cells without destroying them seemed like science fiction. Now it has been done in the lab, and the door has been nudged open for applications in regenerative medicine. Corrected cells could, in theory, be used to grow healthy tissue for transplants, repair organ damage, or even reduce the risk of secondary conditions like heart defects, thyroid disorders, and Alzheimer’s disease — all of which are more common in people with Down syndrome. The potential is wide, stretching from medical interventions to new ways of studying the condition in controlled laboratory settings.
Yet, every scientific leap comes tethered to its own constraints. This technique is far from ready for hospitals, as researchers like Hashizume emphasize. The procedure must be refined to ensure precision — eliminating the extra chromosome without accidentally harming healthy ones — and to make sure that cells remain stable and functional over time. Gene editing’s power cuts both ways: its ability to rewrite biology is matched by the risks of unintended changes, which could lead to new health problems if not carefully managed. Researchers are now fine-tuning the CRISPR guides to lock in on the extra chromosome 21 with greater accuracy, reducing off-target cuts that could undermine the safety of future therapies.
Even if perfected in the lab, bringing such an approach into clinical reality faces steep challenges. Introducing CRISPR into the right cells inside the human body, ensuring the edits hold over decades, and navigating complex regulatory pathways will take years — possibly decades — of additional work. The leap from Petri dish to patient is not a matter of scaling up; it’s a matter of rewriting the rules for what medicine can do at the deepest level. The promise is immense, but so is the journey ahead.
The Ethical Crossroads
As the technical frontier expands, so does the ethical one. Editing out the genetic cause of Down syndrome inevitably raises questions about how society values difference, diversity, and human variation. In places like Iceland, Down syndrome has become rare, not through genetic editing, but due to widespread prenatal screening and selective abortion — a trend that has sparked international debate. Critics worry that similar technologies, if brought into mainstream use, could be driven not only by the desire to ease medical burdens but also by a societal pressure toward genetic conformity. Astridur Stefansdottir, a medical doctor and professor in applied ethics at the University of Iceland, has noted that people with Down syndrome and their families often find such developments unsettling, fearing the erosion of acceptance and the richness of human diversity.
For scientists like Hashizume, the goal is not to erase Down syndrome from existence but to better understand and, where possible, alleviate the physical challenges it brings. Still, intent and impact are not always the same. A technology powerful enough to delete a chromosome is powerful enough to reshape the choices parents make, the way healthcare systems counsel families, and how society frames what is “normal.” If unregulated, the line between therapeutic use and eugenics could blur in ways that would be difficult to reverse.
This is why many bioethicists argue for public discussion alongside scientific development, ensuring that the voices of people with Down syndrome, their families, and disability advocates are part of the conversation from the very start. Science does not happen in a vacuum; it unfolds within a cultural and moral context. How we use it will reflect not only our technological abilities but also our collective values. The breakthrough in Japan is a reminder that innovation and introspection must advance together.

A Wider Lens on Progress
Breakthroughs like this force us to reimagine what is possible — not just for a single condition, but for the future of medicine. CRISPR began as a way to make precise cuts in DNA; now it has been used to erase an entire chromosome. That’s a shift in scale that expands the boundaries of biology itself. It challenges the fatalism that sometimes surrounds genetic disorders, offering a vision — still distant, but tangible — of change at the most fundamental level.
But technological power always comes with a mirror. What do we, as a society, want to change? Which conditions do we see as illnesses to be cured, and which as variations to be embraced? The answer cannot come from science alone. It must come from listening to the people whose lives will be most affected, weighing the potential for relief against the value of diversity, and making space for both hope and humility in the conversation.

If there’s a takeaway from this achievement, it’s that progress is never just about what we can do — it’s about what we choose to do with it. The scientists at Mie University have shown us a new doorway, one that could lead to remarkable healing or troubling uniformity depending on how we walk through it. And perhaps the real measure of advancement will not be whether we can remove an extra chromosome, but whether we can carry forward the compassion, wisdom, and inclusivity needed to use that power wisely.
Loading...

