A Single Treatment Eliminated Every Breast Cancer Tumor Tested

For many people diagnosed with breast cancer, treatment is not a single event but a long, exhausting chapter of life. It often begins with shock and fear, followed by surgery, radiation, chemotherapy, hormone therapy, and years of follow-up appointments. Even when doctors declare treatment successful, many patients live with an ongoing sense of uncertainty, wondering whether the cancer will return.
That fear is especially intense for people diagnosed with aggressive forms of breast cancer. For them, the margin for error is smaller, treatment options are fewer, and recurrence can happen quickly. It is within this emotional and medical reality that a new discovery from researchers at UCLA has begun attracting widespread attention.
Scientists there have developed an experimental immunotherapy that eliminated cancer cells in every breast tumor sample it was tested on, including samples from patients with late-stage triple-negative breast cancer. While the treatment has not yet been tested in humans, the results have prompted cautious optimism and renewed discussion about what the future of cancer treatment could look like.
What makes the finding particularly striking is not only its apparent effectiveness, but also its accessibility. Instead of requiring a personalized manufacturing process for each patient, the therapy is designed as a single, off-the-shelf product that could be used broadly and potentially across multiple cancer types.
Why Breast Cancer is Not One Disease
Breast cancer is often spoken about as though it is a single illness, but in reality it is a broad category that includes many biologically distinct diseases. Tumors can behave very differently depending on their molecular makeup, growth rate, and ability to evade treatment.
Some breast cancers grow slowly and respond well to hormone-blocking therapies. Others are fast-growing, resistant to treatment, and more likely to spread beyond the breast. These differences influence everything from treatment decisions to long-term outcomes.
Triple-negative breast cancer, often abbreviated as TNBC, represents one of the most challenging subtypes. The name itself explains much of the problem. These tumors lack estrogen receptors, progesterone receptors, and excess HER2 protein, which are the three main targets used in modern breast cancer therapies.
Because these targets are missing, many of the most effective drugs simply do not work. Chemotherapy remains the primary option, despite its well-known side effects and the physical toll it can take on patients. TNBC also tends to affect younger individuals and is more likely to recur within the first few years after treatment.
The Persistent Threat of Recurrence

For many breast cancer survivors, the end of treatment does not bring immediate relief. Cancer recurrence can happen months or even years after initial therapy. Recurrence may be local, regional, or distant, with metastatic disease being the most difficult to treat.
Aggressive cancers such as triple-negative breast cancer and inflammatory breast cancer have higher recurrence rates than other subtypes. Factors like younger age at diagnosis, higher cancer stage, and tumor biology all play a role.
Recurrence happens when even a small number of cancer cells survive treatment. These cells can remain dormant and undetectable by imaging or blood tests before eventually growing again. When cancer returns, it is often harder to treat than the original disease.
This reality underscores why researchers place such importance on therapies that aim to eliminate cancer cells completely rather than simply shrinking tumors.
The Rise of Immunotherapy and Its Limits

Over the past decade, immunotherapy has reshaped cancer treatment. Instead of directly attacking tumors with chemicals or radiation, immunotherapy works by harnessing the body’s own immune system.
One of the most well-known examples is CAR-T cell therapy. In this approach, doctors collect immune cells from a patient, genetically modify them to recognize cancer cells, and then reinfuse them. CAR-T therapies have produced dramatic results in certain blood cancers.
However, success in blood cancers has not translated easily to solid tumors like breast cancer. Solid tumors are complex and constantly evolving. They contain many different cell types and can change their surface markers to avoid detection.
In addition, solid tumors create a protective environment around themselves. They recruit cells that suppress immune responses, effectively shielding the tumor from attack. These barriers have limited the effectiveness of many immunotherapies.
The Logistical Challenge of Personalized Treatment
Beyond biology, there are practical challenges that limit access to advanced immunotherapies. Personalized treatments like CAR-T therapy require collecting a patient’s cells, shipping them to specialized facilities, modifying them, and then sending them back for infusion.
This process can take weeks. For patients with fast-moving cancers, delays can mean disease progression. The cost is also significant, often exceeding $100,000 per treatment, placing enormous strain on healthcare systems and patients alike.
These limitations have led researchers to ask whether powerful immune-based therapies could be developed in a way that is faster, simpler, and more affordable.
A Different Immune Cell Offers a New Path
The UCLA research team approached the problem from a different angle by focusing on a rare immune cell known as an invariant natural killer T cell, or NKT cell. These cells occupy a unique space in the immune system, sharing features of both T cells and natural killer cells.
NKT cells are capable of responding rapidly to danger signals and coordinating broader immune responses. Importantly, they can function across different immune systems without triggering severe immune rejection.
Researchers engineered these NKT cells with a chimeric antigen receptor that targets mesothelin, a protein found at high levels on triple-negative breast cancer cells and other aggressive tumors.
The resulting therapy, known as CAR-NKT cell therapy, was designed to address both the biological defenses of solid tumors and the logistical barriers of personalized treatment.
How CAR-NKT Therapy Attacks Cancer on Multiple Fronts
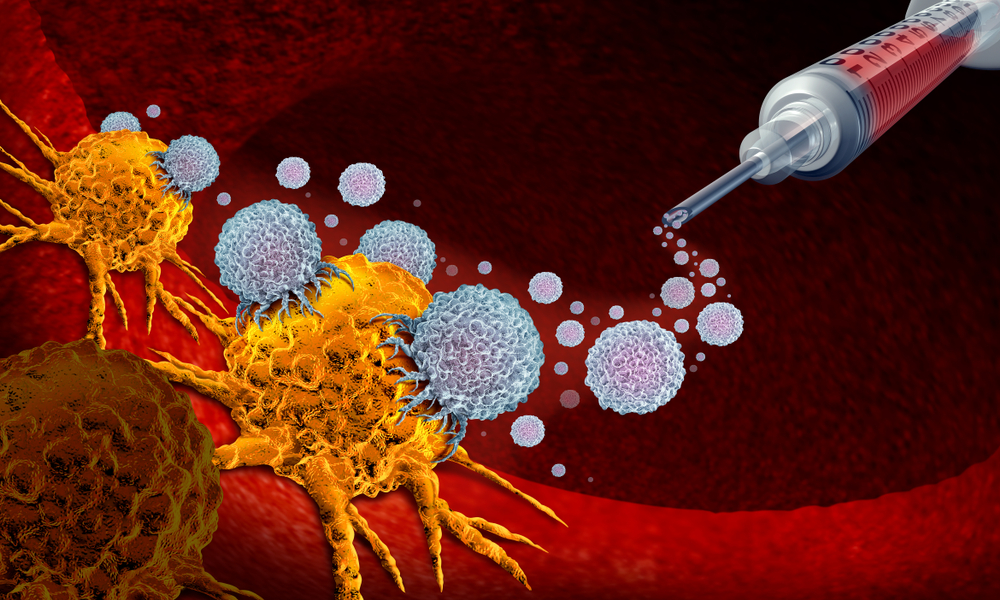
According to the researchers, CAR-NKT cells attack tumors through three distinct mechanisms working simultaneously.
First, the engineered receptor allows the cells to directly recognize and kill cancer cells expressing mesothelin.
Second, the natural receptors carried by NKT cells can recognize more than 20 different molecular stress signals. This makes it extremely difficult for tumors to evade detection by altering a single marker.
Third, NKT cells can reshape the tumor microenvironment. Many tumors protect themselves by recruiting immunosuppressive cells that weaken immune responses. CAR-NKT cells eliminate these protective cells, stripping tumors of their defenses.
Rather than relying on a single point of attack, the therapy overwhelms cancer from multiple directions at once.
Laboratory Results That Drew Global Attention
To test the therapy, researchers applied CAR-NKT cells to tumor samples taken from patients with late-stage metastatic triple-negative breast cancer. These samples represented some of the most aggressive disease encountered in clinical settings.
In every tumor sample tested, the CAR-NKT cells successfully killed the cancer cells. They also removed the immunosuppressive cells that normally shield tumors from immune attack.
While laboratory results do not guarantee success in human patients, the consistency of these findings stood out to many scientists. Seeing a therapy perform uniformly across samples from different individuals is uncommon in cancer research.

Making Cutting-Edge Treatment More Accessible
One of the most significant aspects of CAR-NKT therapy is how it is manufactured. Instead of using each patient’s own cells, the therapy can be mass-produced from donated blood stem cells.
Because NKT cells naturally work across immune systems, a single donation could generate enough cells for thousands of treatments. Researchers estimate that this approach could reduce the cost per dose to around $5,000.
Equally important, the therapy could be stored and ready for immediate use. For patients facing aggressive cancer, eliminating weeks-long manufacturing delays could make a meaningful difference.
A Platform With Broader Implications
The potential applications of CAR-NKT therapy extend beyond breast cancer. Mesothelin is also highly expressed in ovarian, pancreatic, and lung cancers, which are among the most difficult cancers to treat.
Because the same CAR-NKT cell product could theoretically be used across multiple cancer types, researchers describe the therapy as a platform rather than a single-use solution. This could significantly shorten development timelines for future treatments.
Preclinical testing for both triple-negative breast cancer and ovarian cancer has already been completed. The research team is now preparing applications to begin clinical trials.
Parallel Breakthroughs Shaping the Future
The UCLA discovery is part of a broader shift in cancer research. In a separate line of research, scientists have reported experimental small-molecule drugs that eliminated or dramatically shrank breast tumors in mice after a single dose.
These drugs target estrogen receptor positive breast cancer, which is biologically different from triple-negative disease. Still, the findings suggest a shared goal across research efforts: killing cancer more precisely while minimizing long-term harm.
Shorter, more targeted treatments could reduce side effects, improve quality of life, and lower the emotional burden of prolonged therapy.
What Clinical Trials Must Answer
Despite the excitement, researchers emphasize that CAR-NKT therapy remains experimental. Clinical trials will need to answer critical questions about safety, dosing, durability, and effectiveness in living patients.
Human immune systems are complex, and laboratory success does not always translate to clinical success. There are also regulatory and manufacturing hurdles that must be cleared before any new cell therapy becomes widely available.
Reaching the clinical trial stage, however, represents a major milestone. It reflects years of preclinical testing and sufficient evidence to justify testing the therapy in people.
What This Could Mean for Patients and Families
If CAR-NKT therapy proves successful in clinical trials, it could reshape breast cancer treatment on multiple levels.
Patients could receive advanced immunotherapy without long waiting periods. Healthcare systems could offer cutting-edge care at a fraction of current costs. Doctors could potentially use a single therapy across multiple aggressive cancer types.
For families navigating a cancer diagnosis, such changes could translate into more options and fewer impossible decisions.
Living With Uncertainty While Science Advances
Cancer research has taught both scientists and patients to balance hope with realism. Many promising treatments have failed in later stages of testing. At the same time, progress has been steady and cumulative.
The finding that a single breast cancer treatment eliminated every tumor tested in the laboratory does not mean a cure has arrived. But it does suggest that long-standing barriers in cancer treatment may be starting to fall.
It points toward a future where therapies are faster, broader, more affordable, and designed with both biology and access in mind.
A Cautious but Meaningful Step Forward
For patients who have experienced recurrence or limited treatment options, even cautious progress matters. It represents movement in a field where time is often measured in scans, treatments, and waiting.
As CAR-NKT therapy moves closer to clinical trials, researchers, clinicians, and patients will be watching closely. Whether it ultimately fulfills its promise remains to be seen.
What is already clear is that the approach has opened a new path in cancer research. It challenges long-held assumptions about how immunotherapy must be designed and delivered.
In a landscape shaped by uncertainty, that alone is a meaningful development.
Loading...

