Three Scientists Win Nobel for Finding Immune System’s Hidden Security Guards

Your body creates weapons that could destroy you from the inside. Every single day, biological processes generate billions of cells designed to attack and kill. Random genetic combinations inevitably produce defenders programmed to target your own organs, tissues, and blood. Yet most people never experience this internal warfare.
Scientists spent decades puzzled by this paradox. How does our immune system distinguish friend from foe when invaders camouflage themselves to look like human cells? Why don’t we all suffer devastating autoimmune diseases when our bodies create so many potentially self-destructive immune cells? Something fundamental about human biology remained hidden despite centuries of medical research.
Three scientists just received the Nobel Prize in Physiology or Medicine for solving this ancient mystery. Their discoveries revealed security guards operating inside every healthy person, preventing immune systems from launching friendly fire attacks. What they found launched an entire field of medicine and promises treatments for diseases that have plagued humanity forever.
Your Body Creates Billions of Cells That Could Kill You
BREAKING NEWS
The 2025 #NobelPrize in Physiology or Medicine has been awarded to Mary E. Brunkow, Fred Ramsdell and Shimon Sakaguchi “for their discoveries concerning peripheral immune tolerance.” pic.twitter.com/nhjxJSoZEr— The Nobel Prize (@NobelPrize) October 6, 2025
Human immune systems generate more than one quadrillion different T cell receptor combinations through random genetic mixing. Each receptor acts like a unique sensor scanning for threats among thousands of microbes attempting daily invasion. Such diversity ensures defenses exist against every possible pathogen, including viruses humanity has never encountered.
Random combination processes work brilliantly for defense but create dangerous side effects. Inevitable mathematical probability means some T cell receptors will recognize and attach to proteins from human organs, bones, skin, and blood vessels. These self-recognizing cells possess power to trigger devastating tissue destruction across entire organ systems.
Microbes evolved camouflage strategies that mimic human cellular markers, making friend-foe distinction even more challenging. Invaders hide behind molecular disguises resembling normal body components. Immune systems must somehow identify genuine threats while ignoring trillions of friendly cells despite sophisticated enemy deception.
Mystery deepened when researchers discovered that testing mechanisms they understood couldn’t possibly account for how rarely autoimmune catastrophes occur. Something else must be protecting people from their own defenses.
Three Scientists Just Won Nobel Prize for Solving This Puzzle

Mary Brunkow from Institute for Systems Biology in Seattle, Fred Ramsdell at Sonoma Biotherapeutics, and Shimon Sakaguchi from Osaka University received the 2025 Nobel Prize for discovering regulatory T cells. These immune system security guards patrol constantly, monitoring other immune cells and preventing attacks on body’s own tissues.
Nobel Committee Chair Olle Kämpe explained the significance: “Their discoveries have been decisive for our understanding of how the immune system functions and why we do not all develop serious autoimmune diseases.”
Research launched entire field of peripheral immune tolerance, revealing that immune regulation involves far more complexity than eliminating dangerous cells during development. Protection requires active, ongoing surveillance by specialized cells that most scientists didn’t believe existed three decades ago.
Prize winners will share $1.1 million award recognizing work spanning thirty years across multiple continents. Their perseverance through skepticism and technical challenges transformed immunology and opened pathways toward treatments for cancer, autoimmune diseases, and transplant rejection.
One Scientist Swam Against Entire Field in 1995
Shimon Sakaguchi at Aichi Cancer Center Research Institute in Nagoya, Japan, pursued ideas that contradicted dominant scientific consensus during the 1990s. Most immunologists believed thymus gland eliminated all potentially dangerous immune cells through process called central tolerance during T cell development.
Experiments contradicting this tidy explanation captured Sakaguchi’s attention. Colleagues surgically removed thymus glands from newborn mice expecting weaker immunity from reduced T cell production. Instead, when surgery occurred three days after birth, immune systems went into overdrive, attacking organs and causing devastating autoimmune diseases.
Sakaguchi isolated T cells from healthy mice and injected them into mice without thymus glands. Transferred cells protected recipients from autoimmune destruction, suggesting some T cells actively suppress others from attacking body tissues. Protection couldn’t come from eliminating dangerous cells because those had already matured and entered circulation.
Scientific community remained unconvinced by suppressor cell theories after embarrassing scandals where researchers drew false conclusions from flawed experiments. Field essentially abandoned entire line of investigation, dismissing anyone who claimed protective cells existed. Sakaguchi pressed forward alone despite professional isolation.
Decade-Long Search for Immune System’s Security Guards
Identifying protective cells among billions of similar T cells required finding distinguishing molecular markers. All helper T cells carry CD4 protein on their surfaces, making them indistinguishable through conventional identification methods available during early 1990s.
Sakaguchi needed additional marker that appeared only on protective cells. Ten years of experiments testing different protein combinations finally yielded answer in 1995. Protective T cells carried both CD4 and another protein called CD25 on their surfaces.
Journal of Immunology paper introduced regulatory T cells to world, demonstrating that removing CD25-positive cells from mice triggered autoimmune diseases while adding them back provided protection. Clear evidence showed these cells actively prevent immune system from attacking body’s own tissues.
Skepticism dominated response from immunology community. Researchers demanded more proof before accepting existence of Tregs, as regulatory T cells became known. Field had been burned by suppressor cell hypothesis and refused to repeat past mistakes. Sakaguchi’s vindication would arrive six years later from unexpected source.
Sick Mice from Manhattan Project Hold Missing Clue
Oak Ridge National Laboratory in Tennessee studied radiation effects as part of Manhattan Project developing atomic bomb during 1940s. Researchers noticed some male mice born with unusual condition featuring flaky skin, enormously enlarged spleens and lymph glands, and lifespans measured in weeks rather than years.
Scurfy mouse strain resulted from random mutation on X chromosome. Males carrying single mutated X chromosome developed severe disease while females with two X chromosomes (one healthy) survived and passed mutation to offspring. Pattern indicated single gene malfunction caused immune system rebellion.
By 1990s, scientists understood that scurfy mice died from T cells attacking their own organs. Something about the genetic mutation caused complete breakdown of immune regulation. Molecular biology tools had advanced enough to potentially identify specific gene responsible for disease.
Finding that gene could reveal fundamental mechanisms controlling immune tolerance and provide insights into human autoimmune diseases.
Two Biotech Scientists Hunt for Needle in DNA Haystack
Mary Brunkow and Fred Ramsdell worked at Celltech Chiroscience biotech company in Bothell, Washington, developing pharmaceuticals for autoimmune diseases. Scurfy mice offered potential breakthrough if they could identify mutated gene causing immune dysregulation.
Challenge seemed nearly impossible using 1990s technology. Mouse X chromosome contains approximately 170 million base pair nucleotides. Finding single mutation within that enormous DNA sequence required years of painstaking work with limited molecular biology tools.
Brunkow later described the effort: “It was really a molecular slog, to get to that exact mutation. It was just a very small genetic alteration that results in quite a profound change in the immune system.”
Mapping narrowed possibilities to region containing about 500,000 nucleotides harboring 20 potential genes. Researchers then compared each gene systematically between healthy mice and scurfy mice, searching for differences. Nineteen genes showed no variations. Only twentieth and final gene revealed mutation.
Twentieth Gene Finally Reveals the Answer
Years of dedicated work culminated in 2001 Nature Genetics paper announcing discovery of Foxp3 gene. Previously unknown gene belonged to forkhead box (FOX) family that regulates activity of other genes and affects cell development processes.
Brunkow and Ramsdell suspected rare human autoimmune disease called IPEX might represent human equivalent of scurfy mouse condition. IPEX affects boys through X chromosome mutations, causing skin problems, organ enlargement, and immune system attacks on tissues.
Database search revealed human FOXP3 gene equivalent. Samples collected from affected boys worldwide confirmed harmful mutations in same gene. Discovery explained both mouse disease and devastating human autoimmune condition affecting children.
Laboratories worldwide erupted with activity after publication. Researchers suspected Foxp3 gene might control development of regulatory T cells that Sakaguchi identified six years earlier. Puzzle pieces were falling into place.
Puzzle Pieces Connect in 2003 Breakthrough
Sakaguchi proved two years later that Foxp3 gene controls regulatory T cell development. Gene expresses specifically in protective immune cells. Experiments showed switching on Foxp3 expression could convert ordinary T cells into regulatory T cells with suppressive capabilities.
Ramsdell’s team discovered scurfy mice completely lacked regulatory T cells, explaining why their immune systems attacked organs without restraint. Connection between gene discovery and cell identification became undeniable.
Immunologist Markus Feuerer from Leibniz Institute for Immunotherapy later summarized the significance: “Anyone who does not have an autoimmune disease, that’s because of these regulatory T cells.”
Security guards patrol constantly, monitoring other immune cells and ensuring they tolerate body’s own tissues. After eliminating invading microbes, regulatory T cells calm immune responses so defenses don’t continue operating at maximum intensity indefinitely.
Cancer Tumors Exploit This Protection System
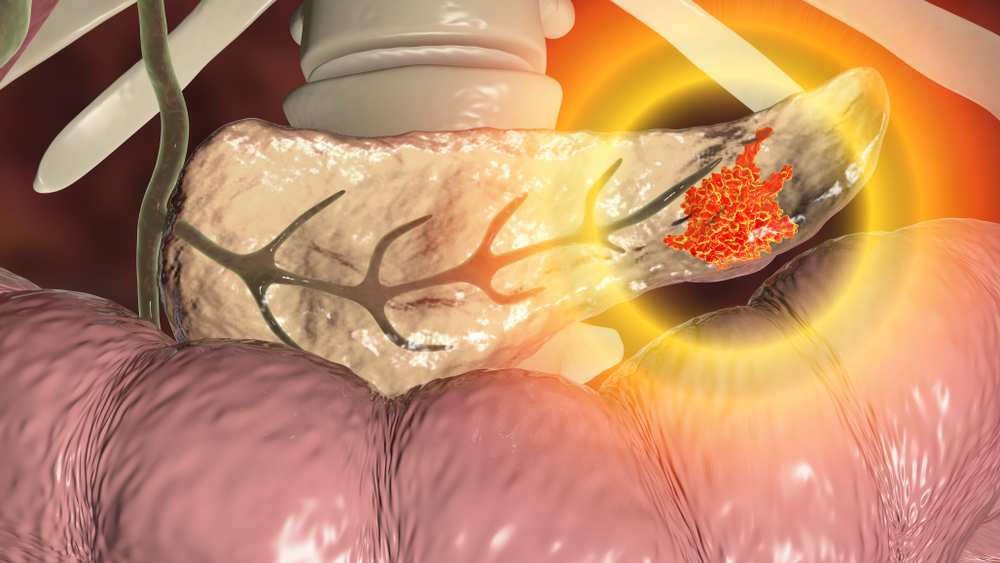
Tumor analysis reveals cancer cells attract massive numbers of regulatory T cells creating protective walls that shield malignancies from immune attack. Cancers essentially hijack security mechanisms designed to prevent autoimmune disease, using them for survival advantage.
Over 200 registered clinical trials currently test strategies for manipulating regulatory T cells. Cancer treatments aim to dismantle Treg shields surrounding tumors, allowing immune systems to access and destroy malignant cells. Research explores ways to reduce Treg activity specifically within tumor microenvironments without triggering autoimmune problems elsewhere.
Cambridge University immunologist Adrian Liston explained the therapeutic potential: “If you control the brakes to the immune system, it gives you the ability to turn the immune response up or to tone it down.”
Opposite approach applies to autoimmune diseases where researchers work to boost regulatory T cell populations. Pilot studies administer interleukin-2, substance that acts like food for Tregs, encouraging their growth and activity. More security guards could calm overactive immune systems attacking joints in rheumatoid arthritis or nerve coverings in multiple sclerosis.
Transplant Patients Could Avoid Lifelong Drugs
Laboratory techniques allow isolating patient’s own regulatory T cells, multiplying them, and returning expanded populations back to body. Modified Tregs can receive antibody address labels directing them toward specific organs requiring protection.
Engineered cells sent to transplanted kidneys or livers could prevent rejection without suppressing entire immune system through dangerous immunosuppressant drugs. Patients might avoid lifelong medication regimens that leave them vulnerable to infections and cancer while still protecting transplanted organs.
Clinical trials currently evaluate whether this approach works in humans after proving successful in animal models. Timeline for widespread availability remains uncertain as regulatory reviews and long-term safety studies take years to complete even for promising therapies.
From Basic Curiosity to Life-Saving Medicine
Discoveries recognized by 2025 Nobel Prize occurred decades before clinical applications became feasible. Sakaguchi’s 1995 regulatory T cell identification and Brunkow-Ramsdell’s 2001 Foxp3 gene discovery laid groundwork that researchers are only now translating into actual treatments.
Blue-sky research driven purely by curiosity about fundamental biological questions ultimately produces breakthroughs that change medicine. Scientists emphasize that supporting basic science remains essential for future discoveries even when practical applications aren’t immediately obvious.
Ramsdell, who co-founded Sonoma Biotherapeutics developing Treg-based therapies, was backpacking off-grid in Idaho wilderness when Nobel Prize was announced. Colleagues couldn’t reach him Monday during official notification attempts. He learned of life-changing news Monday evening after returning from trip.
Brunkow became overcome with emotion at 4 a.m. Seattle announcement, receiving recognition that colleagues noted she didn’t always receive despite contributions. Sakaguchi called award “happy surprise” at Osaka University press conference, thanking students, collaborators, and global research community sharing similar ideas over decades.
Prize winners emphasize their work represents collective effort across many laboratories worldwide, not isolated individual achievements. Science progresses through collaboration and shared pursuit of understanding natural world mysteries.
Loading...

