Tylenol Acetaminophen Pregnancy: Trump Admin Autism Risk Announcement

President Trump stood before crowds this weekend promising a medical breakthrough that would reshape public understanding of autism. Building anticipation for what he called one of the most important announcements of his presidency. Behind closed doors, administration officials prepared to make an unprecedented claim that would pit political leadership against the medical establishment.
Monday’s announcement would mark the first time the U.S. government officially connects a medication used by millions of pregnant women to autism development in children. Yet the scientific community had already begun pushing back, warning that weak evidence could lead to dangerous policy decisions affecting maternal and fetal health across the nation.
Trump Administration Prepares Controversial Medical Announcement
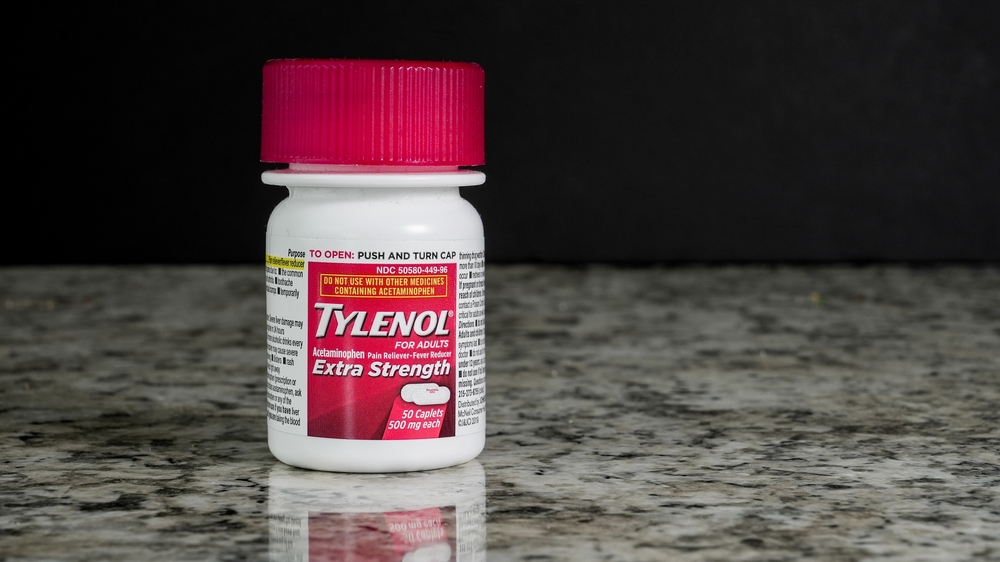
The Trump administration plans to announce that using Tylenol during pregnancy could potentially raise autism risk, according to sources familiar with the preparation. Health and Human Services Secretary Robert F. Kennedy Jr. will lead the effort to advise pregnant women against using the over-the-counter pain medication except for high fevers.
Officials also intend to promote leucovorin, a cancer and anemia drug, as a potential autism treatment despite limited clinical trial data. The dual announcement represents Kennedy’s fulfillment of his April promise to identify autism causes within five months of taking office.
This marks an extraordinary departure from established medical guidance. Acetaminophen, sold under the brand name Tylenol, has been recommended by medical professionals worldwide as one of the few safe pain relief options for pregnant women when used appropriately.
Trump teased the announcement during weekend appearances, claiming his administration had discovered answers to autism that had eluded researchers for decades. Such confident assertions about complex medical conditions typically require extensive peer-reviewed research and professional consensus.
Medical Establishment Rejects Autism Connection Claims

Medical organizations across the United States have preemptively rejected the administration’s planned autism link. The American College of Obstetricians and Gynecologists issued clear guidance defending acetaminophen safety during pregnancy.
International medical authorities echo this position. European health agencies, Canadian medical organizations, and regulatory bodies worldwide continue recommending acetaminophen as a pregnancy-safe pain relief option when used as directed.
Dr. Christopher Zahn, chief of clinical practice for ACOG, emphasized that neurodevelopmental disorders result from multiple factors that cannot be attributed to single causes. Pregnant patients should not be frightened away from safe medications that provide genuine medical benefits.
Scientific Evidence Fails to Support Causal Claims

Despite administration confidence, the scientific foundation for linking Tylenol to autism remains weak and controversial. David Mandell, a University of Pennsylvania professor studying autism, assessed the available research bluntly: “The evidence [from a handful of studies] was really mixed, and the effects were really small.”
Recent studies show modest statistical associations but fail to establish causation. A 2024 Swedish analysis of 2.4 million births initially suggested slight autism risk increases among children whose mothers used acetaminophen during pregnancy. However, when researchers compared siblings where mothers used the medication during one pregnancy but not another, the apparent risk disappeared entirely.
Such sibling comparison studies provide gold standard evidence because they control for genetic and environmental factors that affect entire families. When these controls are applied, the acetaminophen-autism connection vanishes, suggesting other factors explain any observed associations.
Multiple international research teams have attempted to replicate concerning findings without success. The most robust methodologies consistently find no causal relationship between appropriate acetaminophen use and autism development in children.
Research Methodology Problems Undermine Autism Studies
Studies suggesting acetaminophen-autism connections suffer from fundamental methodological flaws that prevent reliable conclusions. Women taking pain medication during pregnancy differ systematically from those who don’t, creating confounding variables that researchers struggle to measure accurately.
Acetaminophen treats fever and pain caused by infections, inflammatory conditions, and other health problems. These underlying conditions independently increase autism risk, making it nearly impossible to separate medication effects from the medical reasons prompting treatment.
Fever during pregnancy, regardless of treatment, raises autism risk by approximately 30% according to large population studies. Researchers have not successfully isolated acetaminophen effects from the infections and inflammatory conditions that necessitate pain relief medication.
Genetic factors create additional complications. Families with autism histories may be more likely to use pain medications due to shared genetic predispositions to inflammatory conditions, creating spurious associations between medication use and autism diagnoses.
Public Health Consequences of Restricting Safe Pain Relief

Current FDA guidance advises pregnant women against using most common pain relievers after 20 weeks due to concerns about low amniotic fluid. Acetaminophen represents one of the few remaining safe options for managing maternal pain and fever during pregnancy.
Untreated maternal fever poses documented risks to fetal development, potentially causing more harm than the medications used to control temperature elevations. Pain management during pregnancy serves legitimate medical purposes that benefit both mothers and developing children.
Former CDC chief medical officer Debra Houry warned about premature policy announcements during a recent press conference: “A press statement that talks about a potential association will cause lots of fear. If there is not the science to back it up, we will see practice changes, worried moms, all sorts of things, and that’s not appropriate. You have to be grounded in gold standard science.”
Restricting acetaminophen access could force pregnant women toward riskier alternatives or leave them suffering through treatable conditions. Either outcome could harm maternal and fetal health more than continued appropriate use of tested medications.
Industry Pressure and Political Timing Raise Questions
LIVE: Pres. Trump Makes Announcement on Massive Medical Findings for American Children – 9/22/25
https://t.co/kcWUsxaucH— RSBN 🇺🇸 (@RSBNetwork) September 22, 2025
Kenvue, Tylenol’s manufacturer, attempted to influence the administration’s announcement through direct lobbying. Company executives met privately with Kennedy to dispute the autism connection and express concerns about consumer confusion resulting from government warnings.
The pharmaceutical industry maintains that decades of safety data support continued acetaminophen use during pregnancy. Global regulatory agencies have reviewed this evidence multiple times since 2014 without issuing autism warnings or changing labeling requirements.
Political timing raises additional concerns about the announcement’s scientific basis. Kennedy promised to identify autism causes within five months of taking office, suggesting predetermined conclusions rather than evidence-driven discoveries. Medical research typically requires years or decades to establish causal relationships.
Trump’s weekend promises of autism breakthroughs followed familiar patterns of extraordinary claims preceding evidence presentation. Such announcements reverse standard scientific practice of peer review and professional consensus before public health recommendations.
Rising Autism Rates Have Well-Documented Explanations
Kennedy frequently describes rising autism diagnoses as an “epidemic” requiring urgent intervention. However, researchers understand the factors driving increased autism recognition without invoking environmental toxins or medication effects.
Diagnostic criteria expanded significantly in 2013 when multiple conditions merged into autism spectrum disorder. Improved screening identifies cases in children and adults whose autism was previously missed or misdiagnosed as intellectual disability or other conditions.
Greater awareness among parents, teachers, and healthcare providers leads to earlier and more frequent referrals for autism evaluation. Adult diagnosis rates increase as people recognize autism characteristics that went unrecognized during childhood decades ago.
These well-documented factors explain autism rate increases without requiring new environmental causes. Population-based studies show autism prevalence remained stable when accounting for diagnostic changes and improved recognition practices.
Professional Medical Community Maintains Evidence Standards

International experts unanimously reject causal claims linking acetaminophen to autism. Professor Ian Douglas from London School of Hygiene and Tropical Medicine warned against “jumping to a causal link” based on observational studies with known methodological limitations.
Durham University researcher Dr. Monique Botha stated definitively that “no robust evidence or convincing studies suggest any causal relationship” between acetaminophen and autism. She described alternative claims as “motivated, under-evidenced, and unsupported by robust methods.”
Professional medical associations worldwide continue recommending acetaminophen as pregnancy-safe when used appropriately. No major health organization has modified guidance based on preliminary research suggesting potential autism connections.
Such professional consensus reflects decades of safety monitoring and rigorous evaluation of available evidence. Medical organizations prioritize patient welfare over political considerations when making treatment recommendations.
Protecting Pregnant Women from Misinformation
Healthcare providers face the challenge of maintaining evidence-based practice while addressing patient concerns about conflicting government messages. Professional medical training emphasizes critical evaluation of research quality rather than acceptance of political announcements.
Pregnant women deserve access to accurate information about medication safety based on the strongest available evidence. Creating unnecessary fear about safe medications could lead to increased maternal and fetal complications when treatable conditions go untreated.
The autism research community continues investigating environmental factors that might influence neurodevelopment. However, such research requires careful methodology, peer review, and replication before informing public health policy recommendations.
Medical decision-making should remain grounded in scientific evidence rather than political timelines or predetermined conclusions. Protecting vulnerable populations requires maintaining professional standards for evaluating treatment safety and efficacy.
Complex medical conditions like autism result from interactions between genetic predisposition and environmental influences that scientists are still working to understand. Simple explanations for complex phenomena rarely prove accurate when subjected to rigorous scientific scrutiny, and pregnant women deserve protection from premature conclusions that could compromise their access to safe, effective medical care.
Loading...

